Bartonellosis in humans
Bartonella species share many general characteristics. Members of the genus are small (approximately 0.3 µm × 1 µm), Gram-negative, pleomorphic coccobacilli. The bacteria are facultative intracellular pathogens, many of which employ hemotrophy (infection of erythrocytes) as a parasitic strategy. All members of the genus are notoriously fastidious and grow slowly in vitro. Bartonella species have been shown to infect a variety of mammalian hosts, and at least three species (B. bacilliformis, B. henselae and B. quintana) are relatively common human pathogens. Vector-mediated transmission is another common procedure within the genus. Bartonella spp. are typically transmitted between mammalian hosts by arthropods, with each bacterial species transmitted by a particular insect vector. Reservoirs for Bartonella spp. include a diverse array of mammals.
Bartonella species have been isolated or detected in cats, dogs, rodents, rabbits and cattle as well as a diverse group of wild animals including wildcats (bobcats, pumas and mountain lions), coyotes, deer, elk and foxes.
At least three species have been identified as major human pathogens, an additional four species, including two subspecies, have been associated with human disease either indirectly or through isolated case reports.
Transmission of Bartonella to humans occurs through an insect vector for most Bartonella species. The list of vectors associated with transmission includes flies, fleas, ticks, lice and mites. The possibility of direct mechanical transmission of B. henselae from cats to humans resulting in cat-scratch disease has been suggested. The role of the cat flea in this process seems likely and may involve the contamination of cat claws with infected flea feces.
Isolation of Bartonella spp. from clinical specimens requires extended incubation times and specialized media.
Bartonella bacilliformis produces an extracellular protein termed “deformin” that can independently generate indentations and trenches in erythrocyte membranes.
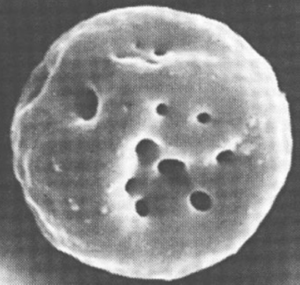
Figure 1. Erythrocyte invaginations caused by B. bacilliformis deformation factor (deformin).
Some Bartonella species produce hemolysins, extracellular proteins that cause incomplete or complete beta-hemolysis, thus potentially the complete destruction of blood cells, resulting into severe anaemia.
The three major pathogenic Bartonella spp. Produce a protein that stimulates angiogenesis and probably facilitates the generation of vascular lesions during infection.
Hemotrophy is a striking aspect of the physiology of most Bartonella spp, which ensures the ideal conditions for life. Heme uptake is employed by several pathogenic bacteria to acquire iron.
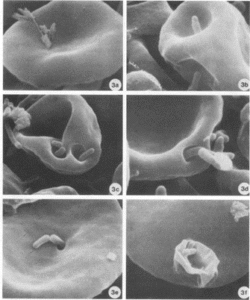
Figure 2. Bartonella bacilliformis invading red blood cells and causing deformations of the surface.

Figure 3. Invaginations on red blood cells potentially caused by Bartonella spp. in a monkey
In addition to invasion of red blood cells, invasion of other host cell types (epithelial and endothelial cells) has been demonstrated for the three major human pathogen species.
Bartonella species evolved strategies to evade detection and degradation by the host immune system, which ensures their proliferation in the host. Following infection, Bartonella alters the initial immunogenic surface-exposed proteins to evade immune recognition via antigen or phase variation. The diverse lipopolysaccharide structures of certain Bartonella species allow them to escape recognition by the host pattern recognition receptors. Additionally, the survival of mature erythrocytes and their resistance to lysosomal fusion further complicate the immune clearance of this species. Certain Bartonella species also evade immune attacks by producing biofilms and anti-inflammatory cytokines and decreasing endothelial cell apoptosis.
Persistent bacteraemia within erythrocytes appears to be a specialized adjustment to the transmission of Bartonella in the host species,
Certain strains of B. henselae exhibit significant gene rearrangements and DNA amplification due to genetic variation, hence DNA-based testing may not always give a positive result.
Clinical presentation of infection with Bartonella ranges from a relatively mild lymphadenopathy with few other symptoms, seen in cat scratch disease, to life-threatening systemic disease in the immunocompromised patient. In some individuals, infection manifests as lesions that exhibit proliferation of endothelial cells and neovascularization, a pathogenic process unique to this genus of bacteria. As the spectrum of disease attributed to Bartonella is further defined, the need for reliable laboratory methods to diagnose infections caused by these unique organisms also increases.
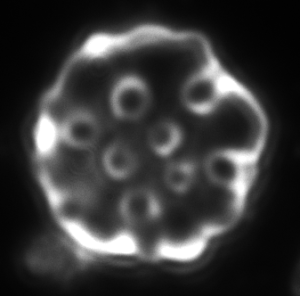
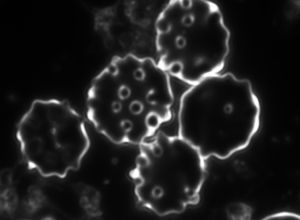
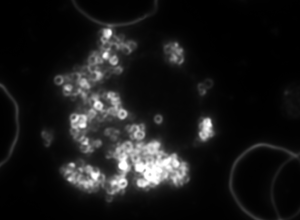
a b c
Figure 4. Dark-field microscopy image of Bartonella infection in a human red blood cells (a-b), and human white blood cell (c)
Due to the changeable genetic profile of Bartonella species, and the lack of knowledge on further human pathogenic Bartonellae, morphological investigations with dark-field microscopy and scanning electron microscopy remain a reliable method of detection.
Further methods used to diagnose Bartonella-associated infections include histopathologic analysis of biopsy specimens, culture of tissue samples, blood culture, and serology, although all of these may show limited success in the clinical setting.
- M Dworkin (Editor-in-Chief): The Prokaryotes, A Handbook on the Biology of Bacteria, third edition, 2006
- Yixuan X et al. Sneaky tactics: Ingenious immune evasion mechanisms of Bartonella, Virulence. 2024
- D H Spach, J E Koehler: Bartonella-associated infections, Infect Dis Clin North Am, 1998
- Anderson BE, Neuman MA.: Bartonella spp. as emerging human pathogens., Clin Microbiol Rev., 1997
- Kaiser PO, et al.: Bartonella spp.: throwing light on uncommon human infections, Int J Med Microbiol., 2011
